Clear your nose and relieve Hayfever fast

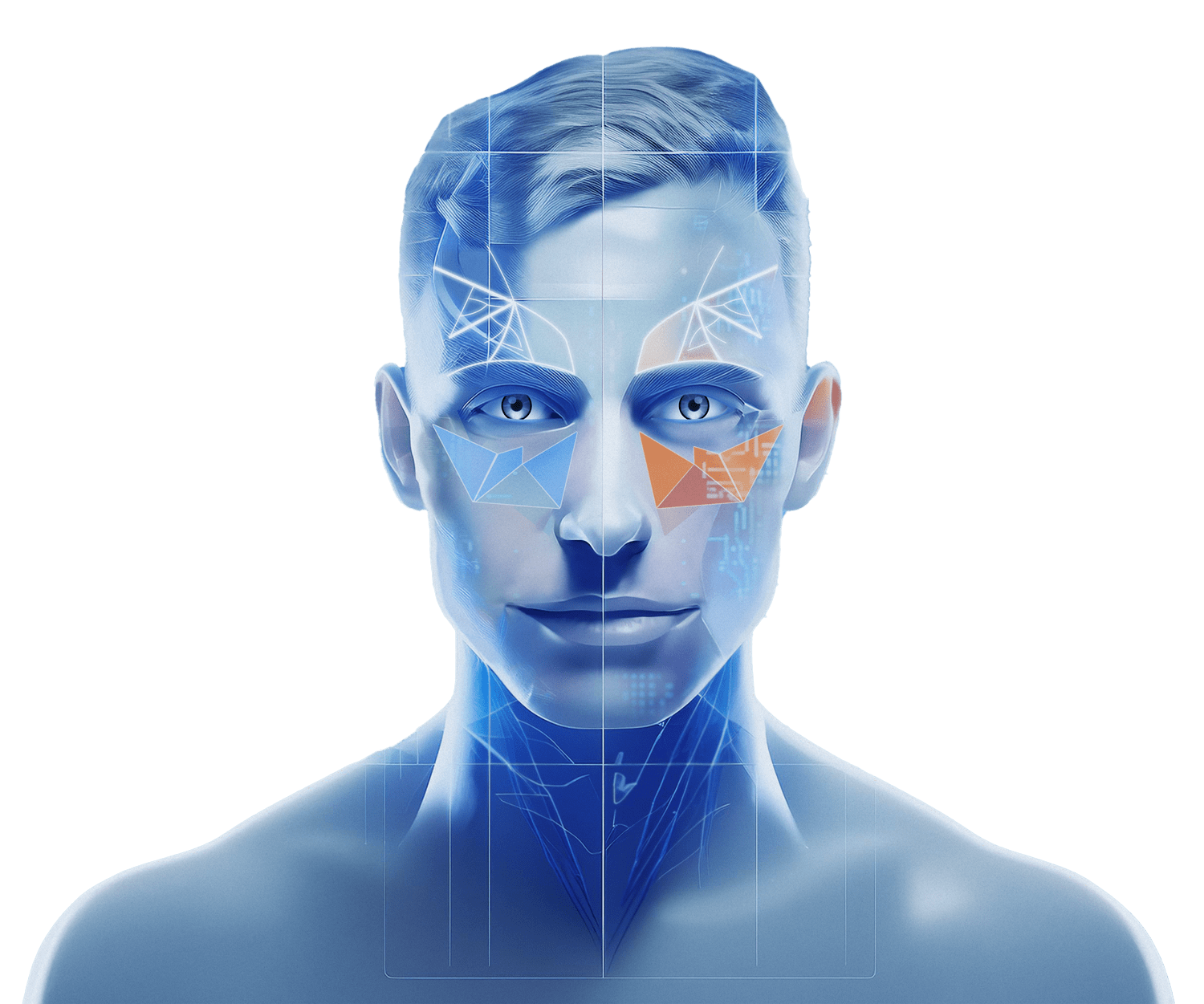

Hayfever and allergic reactions cause the body to release a compound called Histamine. It’s these Histamines that create the common hayfever symptoms of runny nose, watery eyes, itchy skin and sneezing.
Levrix contains levocetirizine, the novel active molecule that treats both seasonal and perennial allergic rhinitis.
Levrix starts working with one tablet giving you fast effective relief for up to 24 hours.
How Levrix works
Levrix works by blocking your body’s Histamine receptors to stop the release of Histamine and allergy symptoms.
The active ingredient in Levrix is called Levocetirizine. It works fast to block Histamine receptors directly at the site where they are needed, giving you targeted strong relief from allergy symptoms for 24 hours.
One clinical study of more than 7000 patients found that 95% of people that try Levrix, stay on Levrix.* That means that 19 out of 20 hayfever sufferers that tried Levrix kept using it.
We’re proud to say that Levrix is also the only Antihistamine tablet with a unique nasal decongestant action.
How to Use
- The usual dose for adults and children aged 6 years and over is one tablet daily.
- Tablets should be swallowed whole with water and may be taken with or without food.
- For a better night’s sleep take Levrix 1 hour before bedtime. You’ll awake refreshed and ready for a new day of allergy relief.
- Always take Levrix exactly as directed. You should check with your doctor or pharmacist if you are not sure.
Always read the label and use as directed. If symptoms persist see your healthcare professional. PHARMACY ONLY MEDICINE. Pharmabroker Sales Ltd, Auckland. TAPS PP3112. *Vos, C. D., Mitchev, K., Pinelli, M., Derde, M., & Boev, R. (2008). Non-Interventional Study Comparing Treatment Satisfaction in Patients Treated with Antihistamines. Clinical Drug Investigation, 28(4), 221-230. doi:10.2165/00044011-200828040-00003 *Potter P.C. Allergy 2003:58:893-899